Blog | 1/14/2020
Bridging the Gap in Clinical and Economic Evidence for Digital Health Tech in the UK
By Anastasios Pappas (Consultant, Health Advances GmbH)
| Summary |
|---|
|
Background
Over the last five years, digital health and biopharma companies have invested heavily in digital technologies. These solutions vary widely in quality and supporting evidence, largely due to the absence of clear standards. Combining this with the minimal barriers to entry for healthcare app developers, the result is a market flooded with low-quality apps and software used by few, if any, patients or clinicians.
Although NICE started evaluating DHTs in 20151, the evidence provided by developers was suboptimal. To improve this, the organization published a framework2 for DHTs intended for use in the NHS.
[1] To date, NICE has evaluated and published five digital app evaluations, which review the strengths and weaknesses of the provided clinical and cost evidence; these are meant to be used as advice, rather than actual recommendation for use.
[2] Evidence Standards Framework for digital health technologies, December 2018, updated in March 2019.
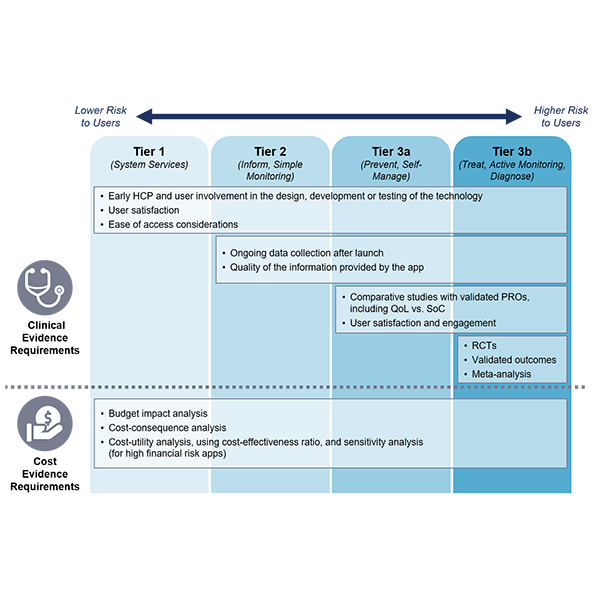
Outline of the Evidence Standards Framework for Digital Health Technologies
NICE released the evidence standards at the end of 2018. The new framework requires two blocks of evidence: clinical effectiveness and economic impact. It also classifies DHTs into three “tiers”, by function, and by potential risk to the users.
- Tier 1: DHTs with no patient outcomes but provide services to the healthcare system
- Tier 2: Information or activities about a condition, and simple monitoring with wearables
- Tier 3a: Facilitation of preventative behavior change like smoking, alcohol, and sexual health
- Tier 3b: Treatment guidance and/or conditions diagnosis
As Figure 1 summarizes, the lower the tier, the lower the evidence requirements.
Figure 1: Select clinical and cost evidence requirements per tier of a digital health technology.
Note: PROs = Patient Reported Outcomes, QoL = Quality of Life, SoC = Standard of Care, RCT = Randomized Clinical Trial.
Source: Health Advances analysis.
Implications for BioPharma Companies
NICE increasingly requires evidence previously applied only to drugs and medical devices, such as controlled studies, PROs, user satisfaction, and cost-effectiveness.
There are several implications for biopharma companies:
- Companies intending to launch DHTs for use in the NHS should assimilate and incorporate the evidence requirements early in the digital solution development plan. This requires more time and investment upfront, but it ensures an easier adoption by NHS post-launch.
- Companies will benefit if they clearly balance the opportunities and drawbacks of evidence generation in each tier. For example, an advanced app feature that leads to more accurate disease diagnosis might not be worth developing if the cost associated with the Tier 3b evidence requirements counterbalances the benefits of having such a feature.
- When selecting digital partners for development, put emphasis on capabilities related to developing clinical grade solutions and generating supporting evidence. In the mid-term, identifying suitable partners will become easier, as new evidence requirements will help make clear which partners have the resources to generate the necessary evidence.
- Getting DHTs used and reimbursed in the NHS will be easier for companies that leverage evidence generation capabilities gained from other areas (i.e., drug development and commercialization).
In the long-run, pharma manufacturers should be encouraged by these new standards. Anything that raises the bar for DHTs will help differentiate high-quality clinical tools from the large volume of low-value apps that have defined much of digital health to date.
References
(1) NICE.
(2) Arnold & Porter 2017 and 2018.
###
| About the Author |
|---|
|
Anastasios Pappas is a member of Health Advances European Practice, which helps clients navigate through the diversity of European healthcare systems to optimize commercialization strategies of pipeline and in-market products. |

