Blog | 8/23/2018
Disease Management Programs (DMPs) in Germany: The Importance of Guideline Inclusion for Chronic Therapies
By Anastasios Pappas (Senior Analyst, Health Advances GmbH)
| Summary |
|---|
|
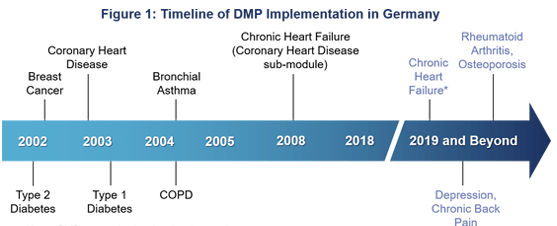
Background There are currently six DMPs in Germany, covering seven chronic disease areas: type 1 and 2 diabetes, bronchial asthma, chronic obstructive pulmonary disease (COPD), coronary heart disease/chronic heart failure and breast cancer. DMPs in Germany only apply to people covered by the statutory health insurance (SHI), which covers approximately ~86% of the German population (71 out of 83 million). DMP History and Current State The first DMP in Germany was implemented in 2002 focusing on type 2 diabetes (see figure 1). With around 4 million members, this DMP is also the largest – accounting for almost 50% of all current DMP participants (see figure 2).
The number of German DMPs will further increase over the next few years, as the chronic heart failure sub-module is set to be separated from the DMP coronary heart disease into a stand-alone DMP. Additional DMPs for depression, chronic back pain, osteoporosis and rheumatoid arthritis are currently in preparation.
Development Process
DMPs are developed by the German Health Authority G-BA1 in collaboration with IQWiG2, are individually approved by the Federal Office of Administration (BVA3), and regionally implemented through SHI and the NAVs4. Within these programs, PCPs act as gatekeepers and are responsible for the enrollment of patients and the coordination of care. While patient enrollment and physician participation are both voluntary, active patient involvement in the DMPs aims to increase therapy compliance.
DMPs are subject to revision every 3 years to ensure the inclusion of the newest guidelines, and IQWiG is typically commissioned by G-BA to perform guideline research as a necessary first step prior to the revision. As an example, the recent revision of the DMP for type 2 diabetes included several newer substance classes, such as GLP-1 agonists as well as inhibitors of DPP-4 and SGLT-2, and strengthened the emphasis on patient age and comorbidities when defining individual HbA1c targets.
Impact of DMPs and Implications
Numerous studies have evaluated the effectiveness of DMPs. For example, the German ELSID5 study, which evaluated the effectiveness of type 2 diabetes DMPs, found that patients in DMPs had a significantly lower overall mortality, higher health-related quality of life and higher treatment satisfaction as compared to patients in the routine care group. Moreover, cost of care per patient was slightly reduced for DMP patients. Similar results have been found in other disease areas or countries. The demonstration of improved patient care and economic savings through DMPs has led to their widespread acceptance and uptake in Germany, and it will be interesting to see whether the DMPs currently under preparation will follow the same path. Since DMPs are developed and revised based on current treatment guidelines, it is important for manufacturers to aim for early guideline inclusion, in order to achieve broader access to large patient populations through these structured programs.
1G-BA: Federal Joint Committee
2IQWiG: Institute for Quality and Efficiency in Health Care
3BVA: Bundesverwaltungsamt (Federal Office of Administration)
4NAV: Association of the Ambulatory Care Physicians
5ELSID: Evaluation of a Large-Scale Implementation of Disease Management Programs
References Health Advances analysis, KBV, BVA, G-BA, Joos 2005 BMC Public Health, Miksch 2010 Am J Manag, Sidorov 2002 Diabetes Care, Fonarow 1997 J Am Coll Cardiol.
About the Author
Anastasios Pappas is a member of Health Advances European Practice, which helps clients navigate through the diversity of European healthcare systems to optimize commercialization strategies of pipeline and in-market products.
Health Advances supports companies with strategies to increase penetration of their products, including through DMPs and other initiatives for creating value-added services for drugs and medical devices.

