Blog | 12/6/2024
The OTC-ing of Medical Devices Marks a Significant Shift in Accessibility
By Brandon Wade, Vice President
For much of the last decade, there was little innovation in OTC medical devices beyond blood pressure monitors. Then came a wave of consumer wearables with tracking capabilities (Apple Watch, FitBit, various rings) and now the last few years have seen FDA OTC clearance for ECG monitors, CGMs, and hearing aids. This trend dramatically impacts patient access, payer considerations, and health tech business models.
Access to CGMs without a prescription will considerably expand the user base with key new segments including health-conscious adults focused on preventative health monitoring and fitness focused users keen to view real-time data from workouts and dietary choices that can integrate with fitness apps.
Along with CGMs, wearable ECG monitors (e.g. AliveCor’s Heart Monitor) will enable users to catch potential issues earlier, leading to significant benefits across key stakeholders: earlier detection for patients, time savings for providers, monetary impact for payers and new revenue channels for health tech players.
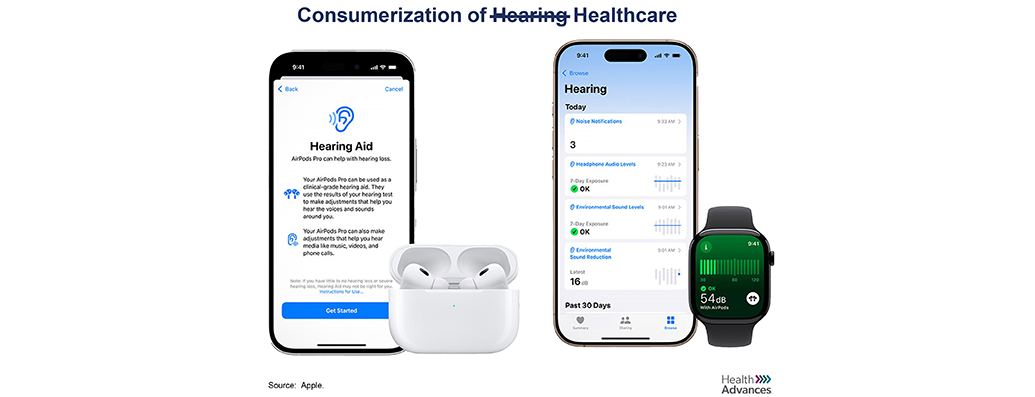
While the 2022 legalization of OTC hearing aids has the potential to meaningfully move the needle on access to hearing loss treatment, the jury is still out on uptake and impact. The FDA estimates that only ~20% of the 30 million US adults living with hearing loss use hearing aids today. OTC devices do not need a prescription or audiologist intervention and enable easy access, but leave patients to their own devices to assess hearing loss and select a product without professional oversight. Could Apple’s AirPods entering the ring at a substantially lower price point change the game? At the pinnacle of design, these devices could go a long way in reducing the stigma associated with hearing aids and pressure incumbents to innovate, overall benefiting patients and the industry as a whole.
As consumers begin to take healthcare data and decisions into their own hands, the need for education on device usage and data interpretation begins to grow. Will health tech players step up to owning this responsibility, or do they need a carrot or stick approach to make that happen?

