Rare Diseases
Development in rare diseases has accelerated dramatically with advancements in genetic research, and new modalities have the potential to transform patient care. That said, most rare disease patients still lack an approved therapy. Challenges facing innovators in the space include small and underdiagnosed patient populations, lack of market precedents, and increasing scrutiny on therapy value.
Health Advances’ involvement in rare diseases dates to the launch of the first enzyme replacement therapies. We have built a strong understanding of the unique dynamics impacting orphan drug development and commercialization and help our clients develop strategies to succeed.
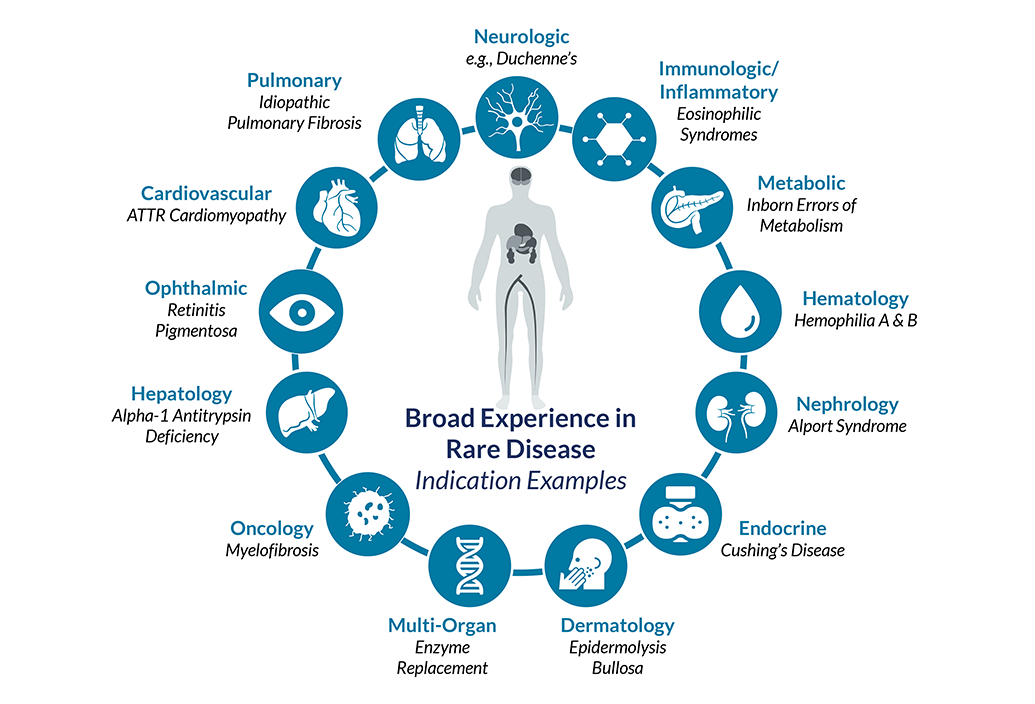
Indications Assessed
Proprietary Rare Disease Analog Database
And other rare disease staff and resources
Rare Diseases Case Studies
Health Advances formed a strategic partnership with a novel biotech to develop the global go-to-market strategy for the company’s first and follow-on products. We recommended the product positioning, prioritized target physician and patient segments, determined the optimal market access pathway and commercial model for >25 countries, evaluated cross-indication pricing dynamics, and developed a life-cycle management and label expansion strategy. The first product has since launched and the company’s valuation increased five-fold over three years.
Our client faced significant challenges to disease diagnosis for its product and needed solutions to improve the patient diagnostic journey. Through our Precision Medicine practice, we shared deep insights from industry analogs and prioritized solutions based on impact on diagnosis and feasibility of implementation. In follow-on work, we determined the ideal features of a novel genotype test, incorporating lab and clinician stakeholder input, and provided a roadmap to rollout test solutions in the US, EU, and Canada.
For a company with a novel gene therapy platform, we performed an indication prioritization leveraging technical reviews and assessments of commercial attractiveness and development feasibility for ~800 monogenic diseases, followed by detailed market opportunity assessments for 15 indications. Our client used the evaluation to prioritize investments and convince investors of the value of its platform.
To inform next steps in the development of a novel CRISPR technology, Health Advances triangulated multiple real-world data sources and secondary resources to arrive at robust patient prevalence and segmentation estimates for a largely uncharacterized disease. Our epidemiology analysis and sales forecast of the asset in the US, EU and Japan gave our client confidence in the business case.
Health Advances performed due diligence on the commercial opportunity for a rare disease asset that resulted in an acquisition. We assessed the likelihood of competitive entry and modeled US and EU erosion curves based on extensive analog analysis. The client subsequently hired our team to do further strategy work to inform near-term market planning and business development activities.