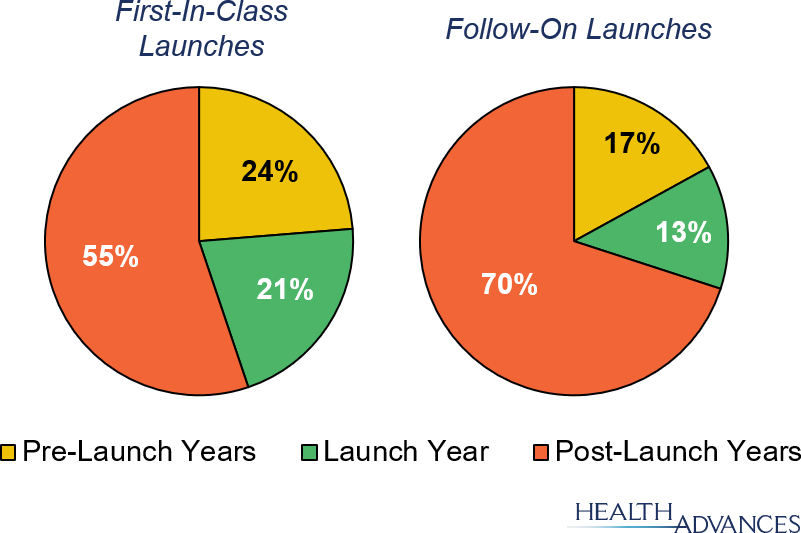
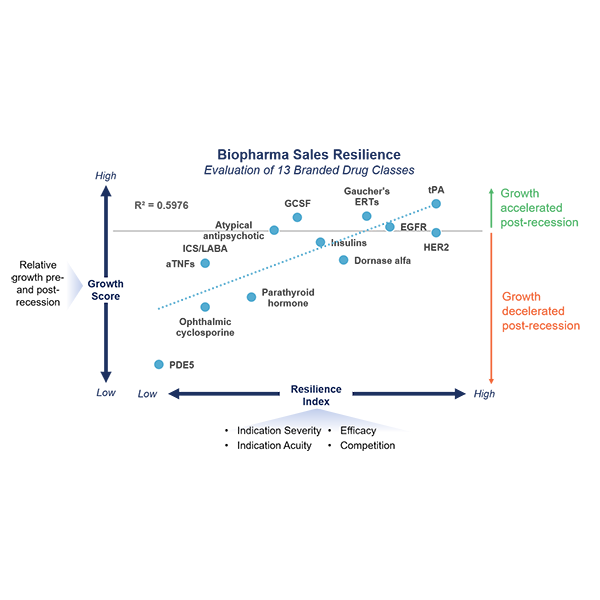
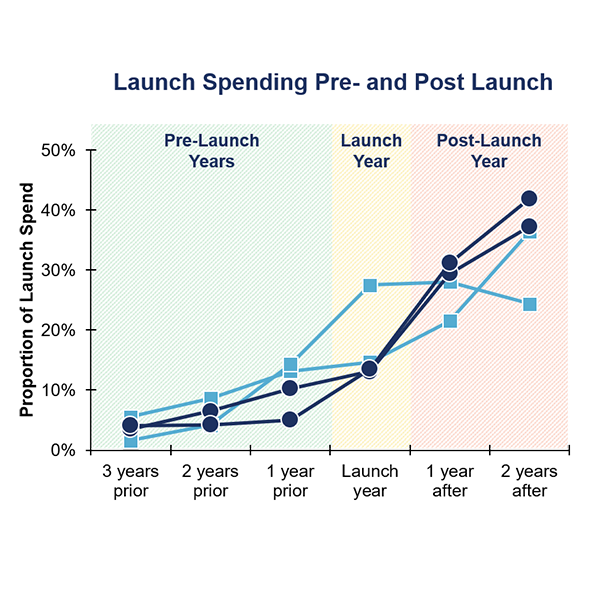
Bioprocessing
Overview
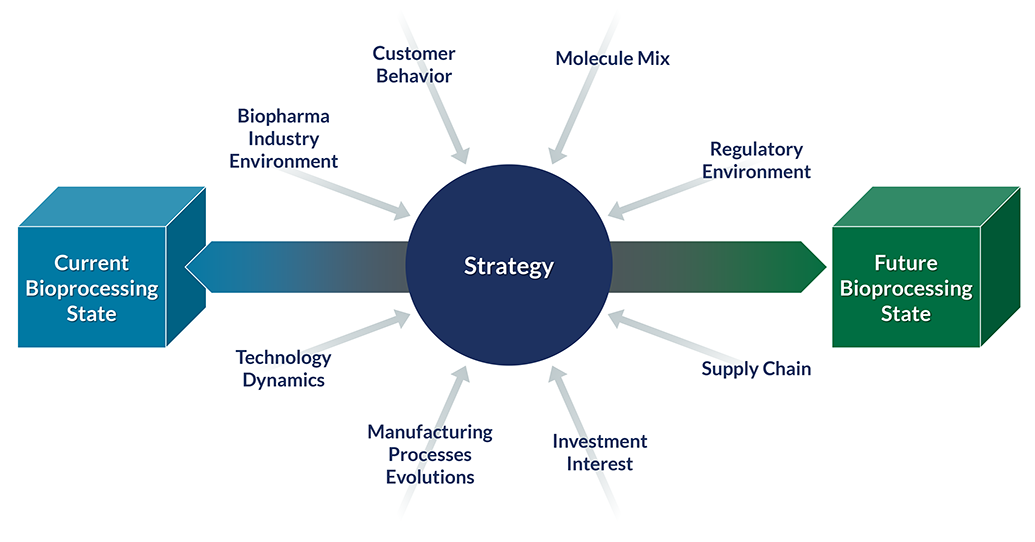
Biologic therapies hold huge promise, from biosimilars that improve access to high-cost treatments, to groundbreaking cell and gene therapies that offer hope to critically ill patients. The manufacturing of these complex therapies, is critical to the function and promise for therapeutic potential. Bioproduction equipment, reagent, and service providers face complex challenges working to define, develop, and scale processes to produce these therapies. Challenges include understanding how processes are evolving, what demand exists in the market, which new technologies need to be adapted, and what positioning will stay ahead of the competition.
We are committed to helping our clients navigate these challenges and capture the unprecedented opportunities to produce these advanced therapies.
Who We Are
Our team of PhDs and MBAs with hands-on experience in biopharma laboratories and GMP production facilities is uniquely positioned to serve clients in the bioprocessing market.
- We understand the complex interplay between the technical, logistical, and business considerations in bioprocessing
- Our bioprocessing team members are dedicated to this area, ensuring we keep up with emerging technologies and trends and our recommendations reflect state-of-the-art approaches
Who We Serve
We collaborate with bioprocessing tools and service companies at all stages to develop business strategies meeting future bioprocessing needs.
- Instrument manufacturers
- Consumables and reagents suppliers
- Software developers
- Cold chain and logistics firms
- CDMOs and other service providers
- Investors
Bioprocessing Case Studies
A global manufacturer and supplier of bioproduction instruments and consumables wanted to ensure it achieved disproportionate growth through developing market-leading solutions for cell and gene therapy manufacturers. Health Advances assessed the types of cell and gene therapies positioned for rapid growth as well as the unit operations with the greatest technical challenges and unmet need and mapped this to the company’s existing capabilities and most accessible technology adjacencies. These findings were utilized to develop a long-term roadmap for product development and identification of high-priority M&A opportunities.
A supplier of reagents primarily used in manufacturing nucleic acids had experienced rapid growth and was interested in exploring opportunities to sustain that growth through building out a robust suite of contract services. Health Advances undertook a multifaceted approach by incorporating direct feedback from conversations with process development and manufacturing experts to understand needs and trends, conducting a survey of potential customers, assessing the competitive landscape of service providers, and identifying key gaps where our client could differentiate themselves. Health Advances provided a summary of the key service offerings that would position them for growth, the technical capabilities needed to develop these service offerings, and ancillary features such as project management that would add value and ensure customer retention.
A large bioprocessing contract development and manufacturing organization engaged Health Advances to evaluate the current performance of its growing protein therapy development business. In partnership with the client, the team developed a detailed interview guide to probe on service provider awareness, selection process, perceptions of the client against its key competitors, and opportunities for the client. The team then recruited and interviewed over 50 hand-selected bioprocessing industry experts across a range of established and emerging biopharma companies and geographies (US, Europe, Asia) with vendor decision-making responsibility. The resulting final report gave the client a customer-centric assessment of its current business performance and clear strategies to grow its business moving forward.
Working with a large US chemicals company, Health Advances evaluated the current and future state of packaging and manufacturing solutions for the pharmaceutical industry, including opportunities across single use consumables for upstream and downstream processing, packaging solutions for pharmaceuticals, materials for contamination control, and materials and logistics solutions for global supply chains. The team conducted interviews with industry experts and supported findings with secondary research. As part of the project, the team developed recommendations around 3 tangible opportunities for further research – enabling next generation perfusion culture, developing next generation filters for optimized depth filtration, and developing novel low-volume 2-8°C shippers for biologics.
A leading private equity and venture capital firm explored an investment in a provider of life science reagents and services for genomics and protein science applications and enlisted Health Advances to validate the attractiveness of the opportunity. Our team evaluated the overall outlook of the market and obtained a comprehensive "outside in" view of market size, market trends, customer segments, competitive dynamics, unmet needs, and value drivers by drawing from discussions with industry experts and published literature. The resulting deliverable helped the client make an investment decision.
Video
9/22/2025
Sharper Strategy Podcast: What’s Next in MRD
This episode is part of Sharper Strategy, a series comprising of Health Advances’ Cross-Sector Insights Across Biopharma, MedTech, Digital Health/HIT, and Diagnostics, Precision Medicine, Life Science Tools & Services.
Learn More
Video

8/7/2025
Sharper Strategy Podcast: Unpacking the Oncology Care Ecosystem
This episode is part of Sharper Strategy, a series comprising of Health Advances’ Cross-Sector Insights Across Biopharma, MedTech, Digital Health/HIT, and Diagnostics, Precision Medicine, Life Science Tools & Services.
Learn More