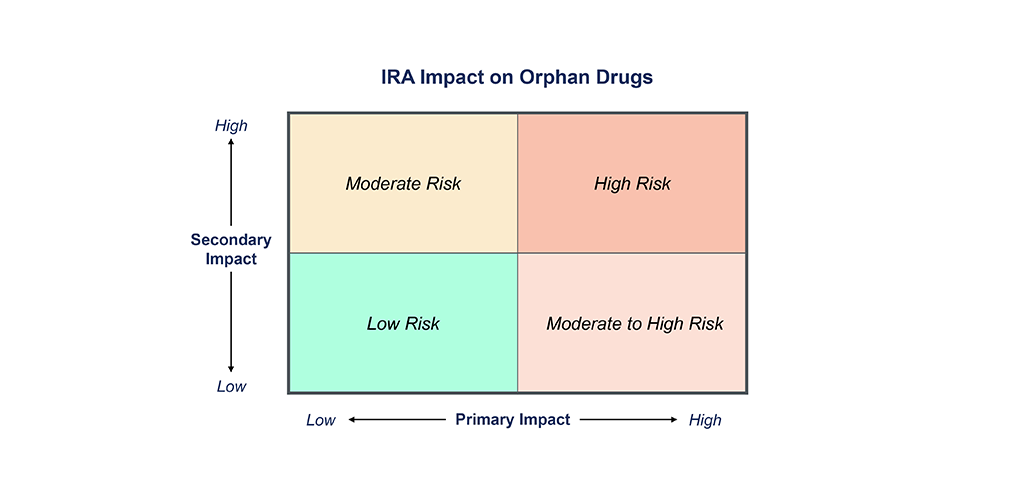
Insights
Health Advances senior leaders and team members are actively engaged in thought leadership across the healthcare spectrum. In our Blog posts and Whitepapers, we comment on a wide range of topics across our five sectors. We speak at conferences, participate in panels, and contribute to specialized journals.